
Shares of Aprea Therapeutics Inc (NASDAQ: APRE) today fell 78.6% after the company announced that the Phase 3 clinical trial evaluating the safety and efficacy of eprenetapopt with azacitidine (AZA) versus AZA alone in TP53 mutant myelodysplastic syndromes (MDS) did not meet the predefined primary endpoint of complete remission (CR) rate.
Investors sold the biotech company’s shares after the release of the disappointing phase 3 clinical trial results earlier today. Today’s data cast a long shadow of doubt on the company’s novel cancer treatments given that this was a phase 3 trial.
In the intention-to-treat population of 154 patients, the CR rate in the eprenetapopt with AZA arm was 33.3% (95% CI: 23.1% – 44.9%) compared to 22.4% (95% CI: 13.6% – 33.4%) in the AZA alone arm (P = 0.13).
The positive aspects of the trial were not statistically significant, but the company promised to perform a deeper analysis of the data by increasing the duration of patient follow up. This data shall be released at a later date.
Dr. Eyal Attar, Aprea’s Chief Medical Officer, said: “Though we are disappointed the topline results did not reach statistical significance, we continue to believe that eprenetapopt can offer clinical benefit to patients with TP53 mutant malignancies,”
Adding:
“We will continue to analyze data as it matures and follow patients who are still receiving study treatment. Our other clinical trials continue to progress and we remain committed to pursuing our clinical development programs.”
Aprea Therapeutics share price
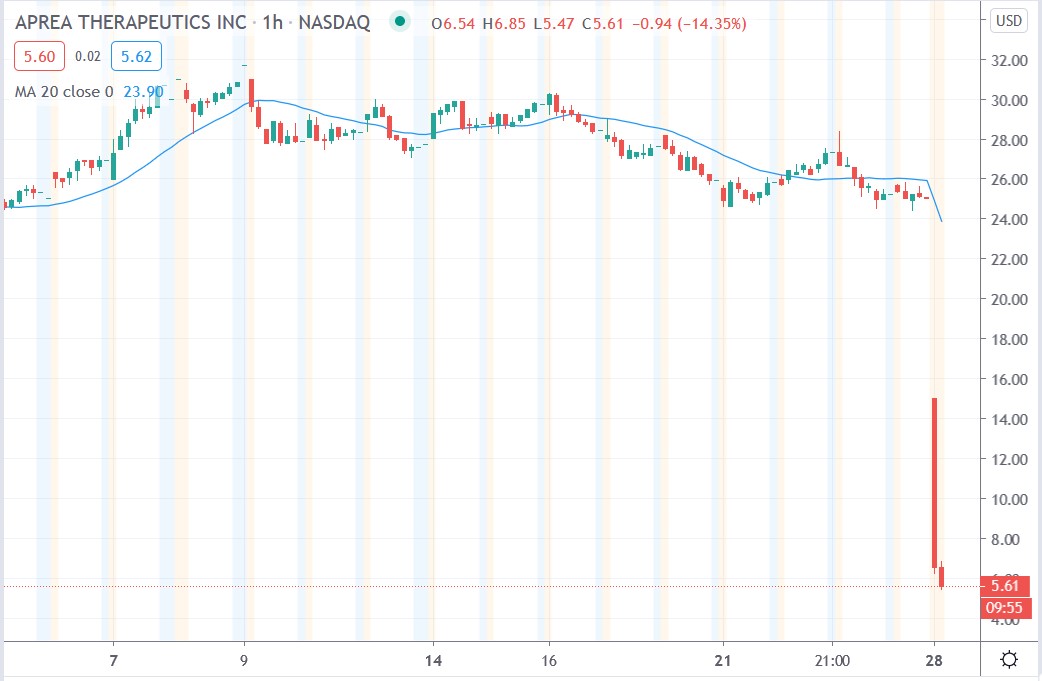
Aprea Therapeutics shares plunged 78.6% to trade at $5.35 having fallen from Thursday’s closing price of $25.09.




