
Shares of biotechnology company Novavax (NASDAQ: NVAX) are creeping higher on Friday after the announcement of progress in its collaboration with Takeda Pharmaceutical Company Limited.
The two firms have signed a licence agreement for Takeda to develop, manufacture and commercialise the NVX-CoV2373, Novavax’ COVID-19 vaccine candidate, in Japan.
Novavax and Takeda are collaborating on manufacturing, clinical development, and regulatory activities in Japan. Novavax will transfer the technology to manufacture the vaccine antigen and supply it's Matrix-M™ adjuvant to Takeda.
Takeda said it anticipates manufacturing over 250 million doses of the Covid-19 vaccine per year, with the company entitled to receive payments based on the achievement of specific development and commercial milestones as well as portions of proceeds from the vaccine.
The Japanese firm has begun a placebo-controlled observer-blinded Phase 1/2 study in Japan to evaluate the drug's immunogenicity and safety.
Novavax has previously reported interim efficacy results in an ongoing Phase 3 trial taking place in the UK.
“It is vital that we extend the reach and availability of vaccines like NVX-CoV2373 to stop the COVID-19 pandemic, and Takeda is well positioned to make that happen,” said Stanley C. Erck, President and Chief Executive Officer of Novavax.
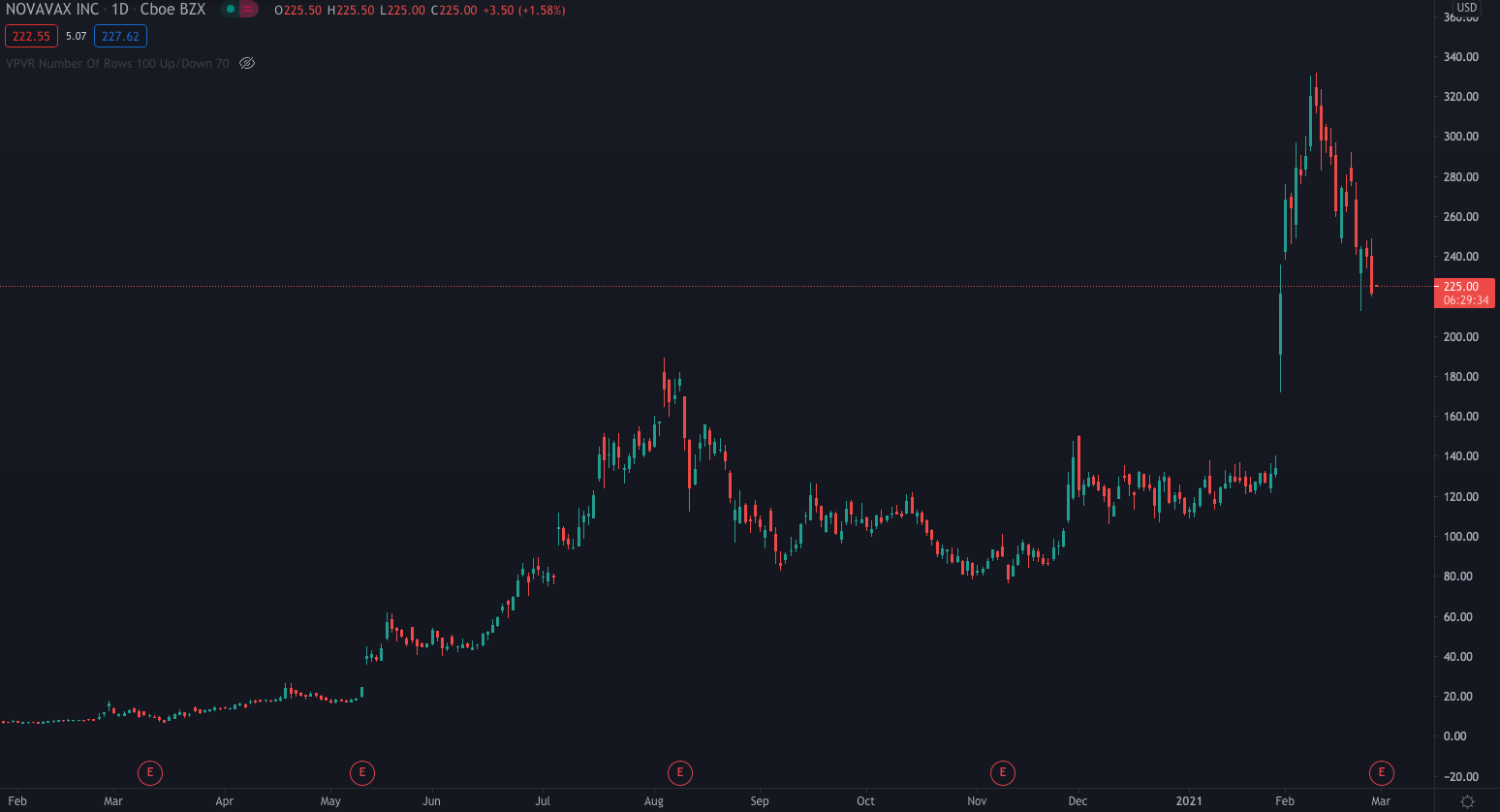
Novavax shares are currently trading 1.58% higher at $225.
