
Shares of Futura Medical plc. (LON: FUM) soared 161.1% after the company’s application for EU’s approval of its erectile dysfunction gel known as MED3000 was recommended for approval by the EU Notified Body.
The recommendation will be followed by the actual EU approval of the topical ED gel under the European Medical Device Regulation, allowing it to be sold across the European Union without the need for a doctor’s prescription.
Futura’s MED3000 gel is based on its proprietary, transdermal DermaSys® drug delivery technology. The company is still working on getting the treatment approved in the US as a non-prescription, clinically proven ED treatment.
Once the device is certified in the EU and granted the CE Mark, Futura will have an easier time getting the drug approved in other countries worldwide. Regions such as the Middle East, Africa, the Far East, and Latin America allow for fast track approval for drugs with the CE Mark.
MED3000 is a differentiated product that works for most patients suffering from ED with different severities. It is well-positioned to carve a niche for itself in the global ED market, which is worth approximately $5.6 billion.
Futura recently received £2 million from HT Riverwood Multi-Growth Fund after partnering with Pride Century Ventures to develop and commercialise MED3000 earlier this month. Today’s recommendation will take the drug a step closer to commercialisation.
James Barder, Futura Medical’s CEO, commented: “The recommendation to approve MED3000 in Europe is a huge milestone for Futura in the development of MED3000. We look forward with excitement to bringing MED3000 to patients in Europe as the first, clinically proven treatment for erectile dysfunction that is highly differentiated with its rapid speed of onset.”
Futura Medical share price.
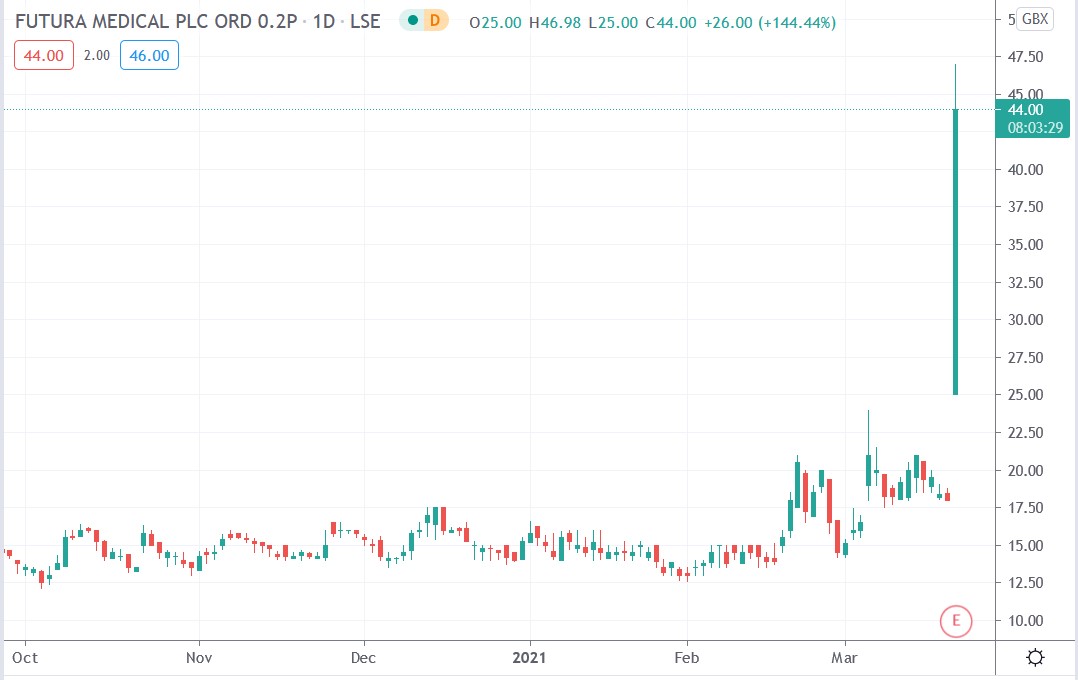
Futura Medical shares surged 88.89% higher to trade at 34p after rising from Thursday’s closing price of 18p.
