The Addex Therapeutics Ltd – ADR (NASDAQ: ADXN) stock price rallied 62.1% after the ADX71149 (JNJ-40411813) Phase 2 epilepsy clinical study was recommended to continue by an Independent Interim Review Committee (IRC) following the review of unblinded data from Part 1 the first patient cohort.
eToro is a multi-asset investment platform. The value of your investments may go up or down. Your capital is at risk.
Investors cheered the news that will see the phase 2 epilepsy study being conducted by Janssen Pharmaceuticals, Inc. proceed. Janssen Pharmaceuticals, Inc. is part of the companies of the same name owned by Johnson & Johnson.
Top Broker Recommendation
- BlackBull 26,000+ Shares, Options, ETFs, Bonds, and other underlying assets – Read our Review
- Admiral Markets More than 4500 stocks & over 200 ETFs available to invest in – Read our Review
- Hargreaves Lansdown The company's website is easily understandable and accessible to a wide range of customers – Read our Review
- eToro Wide range of instruments available to trade – Read our Review
- IG Top-tier regulation – Read our Review
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY
Janssen Pharmaceuticals, Inc. was granted an exclusive worldwide license to develop and commercialise mGlu2 PAM compounds, including ADX71149, by Addex Therapeutics, under the research collaboration and license agreement signed by the two firms.
Addex is eligible for milestone payments of up to €109 million based on the licensed substances' success and meeting critical regulatory milestones. In addition, Addex is eligible for low double-digit royalties on net sales of compounds developed and commercialised under the agreement.
ADX71149 is a selective metabotropic glutamate sub-type 2 (mGlu2) receptor positive allosteric modulator (PAM). The multi-centre Phase 2 study assessed the safety, efficacy, pharmacokinetics and tolerability of adjunctive ADX71149 administered in patients with focal onset seizures with suboptimal response to levetiracetam or brivaracetam.
The study will assess up to 3 doses (low, medium, high) of ADX71149 in up to 160 patients. The study's primary objective is to evaluate the efficacy of ADX71149 when combined with levetiracetam or brivaracetam using a time-to-baseline seizure count endpoint.
Part 1 of the study evaluates the acute efficacy of ADX71149 over four weeks. Participants who do not meet or exceed their monthly baseline seizure count in part 1 continue double-blind treatment in part 2 until they reach their monthly baseline seizure count or eight weeks, which is the maintenance efficacy phase.
Tim Dyer, Addex Therapeutics’ CEO, said: “The recommendation of the IRC and the decision of our collaboration partner to continue the study is very encouraging and suggests ADX71149 is potentially safe and well tolerated and may have a positive impact on this patient population. We look forward to providing further updates on the progression of this important clinical study in the second half of this year.”
*This is not investment advice.
Addex Therapeutics (ADXN) stock price.
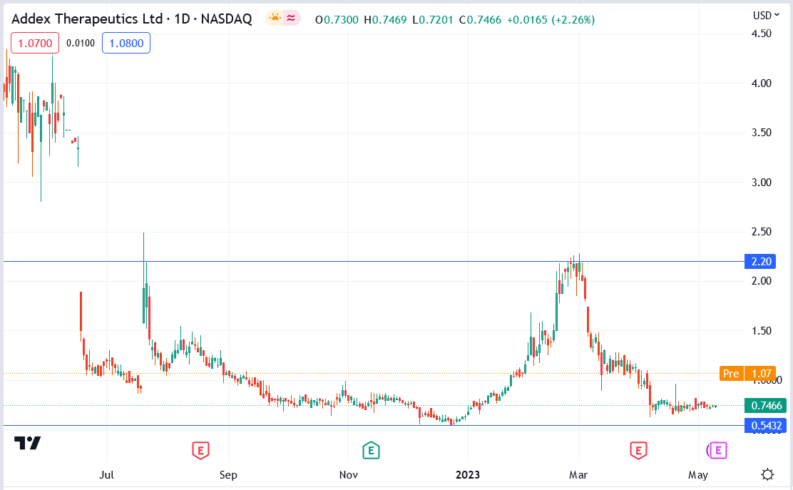
The Addex Therapeutics (ADXN) stock price rallied 62.07% to trade at $1.21, from Tuesday’s closing price of $0.7466.
eToro is a multi-asset investment platform. The value of your investments may go up or down. Your capital is at risk.













