
Shares of 4d Pharma PLC (LON: DDDD) gapped up 10.3% after the company released positive results from its LBP Blautix phase II clinical trial upon further analysis of the data from a diverse sample population.
The company noted statistically significant results on bowel habits among US participants compared to British and Irish patients who had a high response to the placebo drug.
4D Pharma noted that patients receiving LBP Blautix noticed a progressive decrease in abdominal pains during the trial. Their bowel habits also improved significantly as compared to the group that was taking the placebo.
Since March, the biotech company’s shares have been falling, but today’s announcement pushed the shares above 101p resistance level, and I’ll be monitoring the stock from now on to see if the level will hold.
4d Pharma will hold a webcast tomorrow to discuss the results in detail. Investors will be hoping the company’s management will provide timelines on when phase III trials will begin and chart a path towards regulatory approval.
LBP Blautix is the only drug candidate globally that has demonstrated effectiveness against both IBS-C and IBS-D. The treatment could generate significant revenues for the company is commercialised since it could treat all types of irritable bowel syndrome (IBS).
The Phase II trial ran for eight weeks, but the study data analysis has taken much longer. Still, results from US patients indicate that Blautix is twice as effective as the placebo in IBS-D, while its effectiveness in IBS-C is 28.3% vs the placebo at 16.4%.
As traders, we’ll monitor the stock over the next few days to see how it behaves at the current support level.
4d Pharma share price.
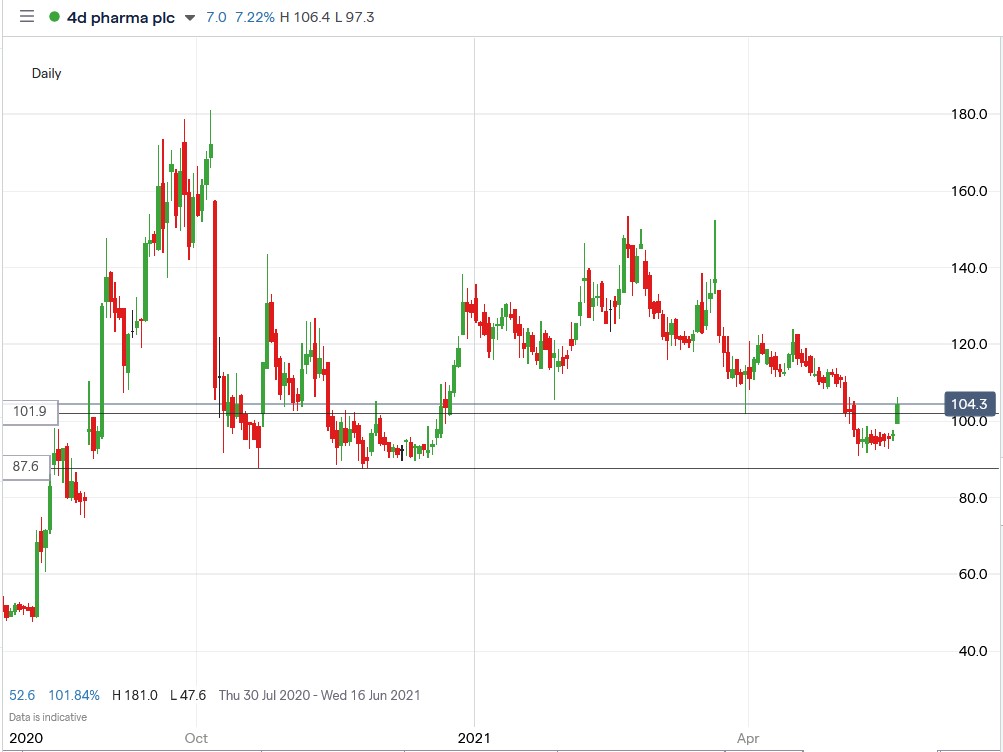
4D Pharma shares surged 10.27% higher to trade at 106.3p, rising from Friday’s closing price of 96.4p.




