Key points:
- Can-Fite has unveiled plans for new patent applications for its Namodenoson cancer treatment.
- The company is likely to be awarded the patents given that its chairman is also the founding partner of a leading IP firm.
- Investors are excited about the firm as they should, but one should never put all their eggs in one basket.
The Can Fite Biopharma ADRs (NYSEAMERICAN: CANF) spiked 36.5% higher after unveiling plans to file patent applications in multiple countries after a patient being treated with its Namodenoson cancer drug achieved a complete recovery.
According to the data released on December 20, 2021, the patient suffering from advanced hepatocellular carcinoma (HCC), the most common form of liver cancer, was cleared of all tumour lesions following treatment with Namodenoson.
The company said that the pending patent applications would add to its growing intellectual property (IP) portfolio based on its drug development platform technology and drug candidates that can treat multiple types of cancers.
Dr Ilan Cohn, Can-Fite’s Chairman, stated: “Can-Fite’s IP portfolio includes about 200 patents and pending patent applications in 16 patent families. Given the very strong new data on Namodenoson’s efficacy in advanced HCC, these latest patent filings seek to further fortify our IP position with respect to Namodenoson in liver cancer and also expand our pending claims for advanced disease in other cancer indications.”
Dr Cohn is also a Founding Partner at Cohn De Vries Stadler & Co., a leading IP firm. Therefore, we can assume that he knows what he is doing regarding IP laws and protection. Given his background, I would expect Can-Fite to win most of its patent applications, if not all of them.
Namodenoson cleared all the lesions in a patient suffering from HCC treated under an Open-Label Extension program of its concluded Phase II study.
Can-Fite intends to start recruiting participants for a phase III clinical trial in Q1 2022 to support a New Drug Application (NDA). The company hopes to gain approval from the US Food & Drug Administration (FDA) and the European Medicines Agency (EMA), who have given the phase III trial the greenlight.
The phase III study will target HCC patients with underlying Child-Pugh B7 (CPB7) cirrhosis.
Namodenoson has been awarded Orphan Drug Designation for HCC by the US and European regulators. The drug also has Fast Track Status in the US.
It appears investors are excited about Can-Fite’s prospects as it applied for new patents to protect Namodenoson as they should. However, we still have a long way to go before the phase III trial is complete, where many are expecting a positive outcome from the extensive study.
However, anything can happen before then, and as prudent investors, we should never put all our eggs in one basket, just in case it falls and we incur massive losses. Therefore, always have a diversified investment portfolio.
*This is not investment advice. Always do your due diligence before making investment decisions.
Can Fite Biopharma stock price.
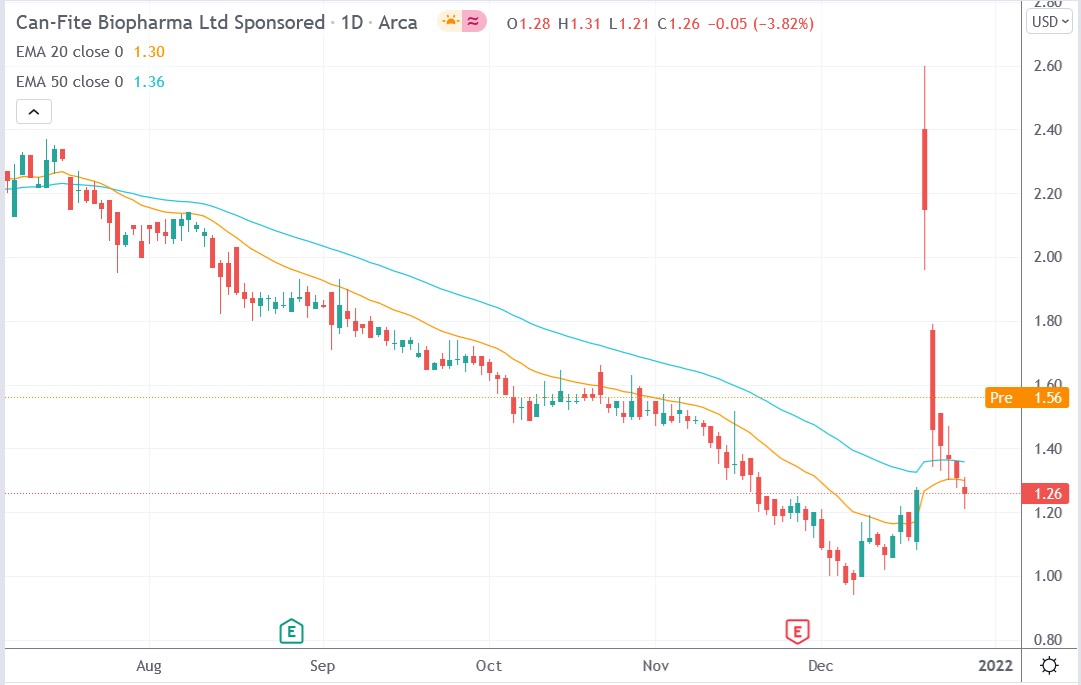
Can Fite Biopharma stock price rallied 36.5% premarket to trade at $1.72, rising from Tuesday’s closing price of $1.26.
Is Now a Good Time to Invest In Can-Fite Shares?
Healthcare stocks, including Can-Fite Biopharma shares, saw a wave of investors buy their shares during the pandemic. Governments also pumped money into the companies in an attempt to speed up the vaccine process. But, what happens now vaccines have been approved and the pandemic is becoming less prominent? Should we still invest in coronavirus-focused healthcare stocks? Or should we look to firms tackling other areas? Here are the best healthcare stocks to buy now…




