Key points:
- Evgen Pharma secured a licensing deal for up to $160.5 million
- The company has secured the licensing deal with Swiss company Stalicla
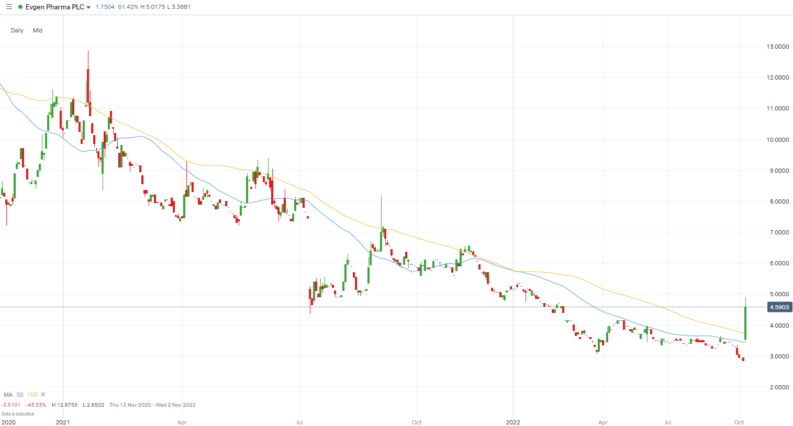
- Evgen Pharma shares surged more than 60% on the news
- key point
Evgen Pharma (LON: EVG), a drug development company, has licensed the global rights in its lead asset SFX-01 in neurodevelopmental disorders and schizophrenia to Swiss group Stalicla, which specialises in the identification of specific phenotypes of autism spectrum disorder.
The partnership of Evgen and Stalicla will enable the targeting of patient groups most likely to benefit from SFX-01, not only de-risking the clinical development but potentially bringing a therapeutic option.
Also Read: Five Best Pharmaceutical Stocks To Watch In 2022
Stalicla will pay $0.5 million upfront and $0.5 million on the completion of the Evgen-sponsored human volunteer Phase 1 study. Furthermore, milestone payments up to commercial launch are $26.5 million. In all scenarios, royalties paid to Evgen on sales will be in the low to the medium double-digit range. In total, the group licensed the asset for approximately $160.5 million if all milestones are met.
“This is an exciting opportunity to work with Stalicla to develop a potential treatment for ASD and other neuropsychiatric disorders,” said Dr. Huw Jones, Evgen CEO.
Jones continued, “There are no current approved treatments for ASD but a molecular target activated by SFX-01 offers considerable promise in alleviating some of the complex behavioural challenges experienced by people with these conditions.”
AIM-listed Evgen shares are up 58% at 4.51p at the time of writing, following the announcement on Monday. The company's shares hit a high of 4.98p earlier in the session, a high last seen in January this year.




