Shares of Mesoblast Limited (ASX: MSB) plunged more than 30% on Tuesday after the Food and Drug Administration (FDA) questioned the evidence of sufficient benefit in the treatment of pediatric patients.
The U.S. regulator released two reports yesterday in relation to Mesoblast’s application for the approval of a stem cell treatment for children with a specific inflammatory condition.
Mesoblast's trial results have successfully reached the primary endpoint of a 28-day overall response rate in patients at 69.1%. The treatment has already received a green light from health authorities in Japan.
The FDA advisory committee is scheduled to meet on Thursday to discuss whether additional clinical trials are required.
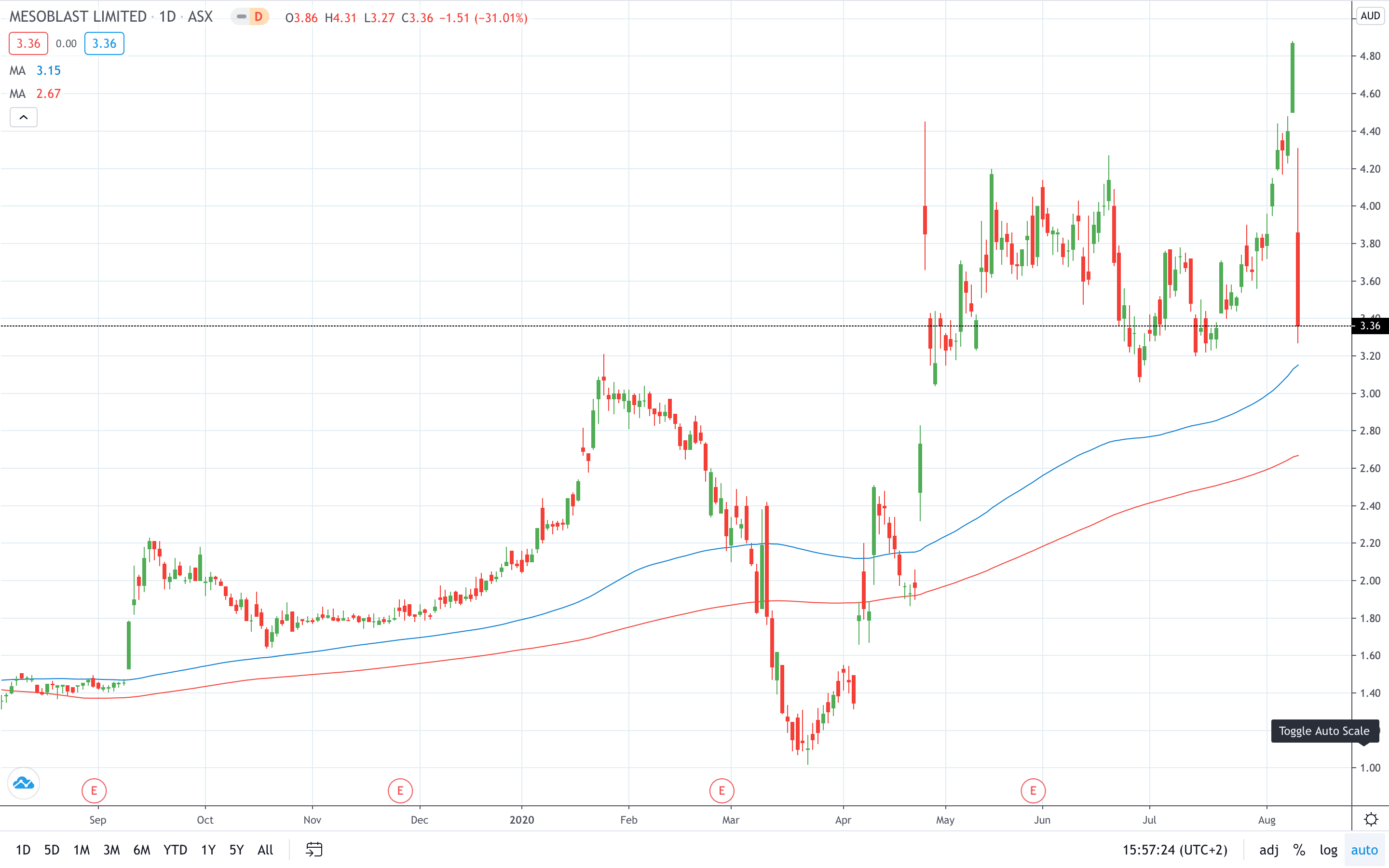
Mesoblast share price plunged over 30% on the news to 3.36, just a day after it logged a 6-year high at 4.88.
- Explore stock trading strategies
- Learn from experts on risk management in trading




