
Shares of ImmuPharma PLC (LON: IMM) today surged 41.9% after the company announced that its US partner Avion Pharmaceuticals had made progress with the US Food & Drug Administration (FDA) in getting its lupus drug approved.
The company noted that the FDA had agreed to a Type-A meeting that would provide clinical guidance for the phase III trial of Lupuzor, the lupus treatment developed by ImmuPharma.
ImmuPharma said that Avion had forwarded the information for the Type-A meeting to the FDA on November 6 and that a meeting usually takes place within 30 days, but this is not guaranteed.
Avion also asked the FDA to consider the conditional approval of Lupuzor before the completion of the final stage clinical trial based on the “clinically significant data” generated from the phase II and III trials, which have been already completed.
The company also confirmed that it had started producing a new batch of Lupuzor for the phase III trial.
ImmuPharma share price
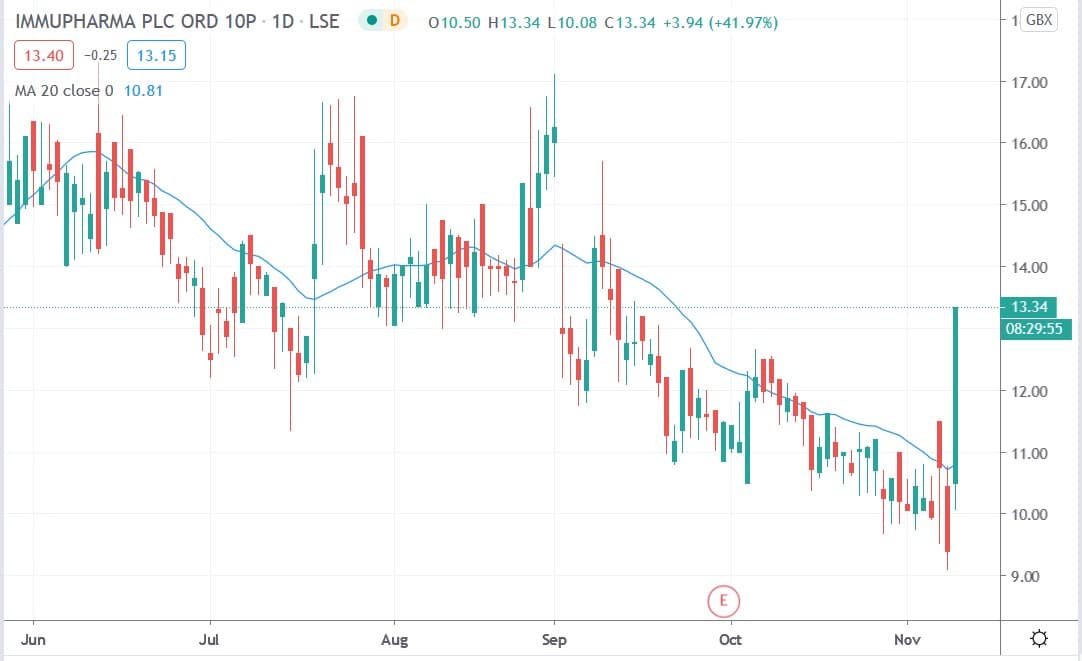
ImmuPharma shares today fell 41.97% to trade at 13.34p having rallied from Monday’s closing price of 9.4p.
People who read this also read:
- Our latest trending stories
- Embrace social trading with eToro
- Here are the 7 best shares to buy in 2020




