

Shares in biopharmaceutical company Ocugen Inc (NASDAQ:OCGN) have shot higher in Monday trading after the company announced that the US Food and Drug Administration (FDA) granted the third orphan drug designation for its OCU400 drug that treats RHO mutation-associated retinal degeneration.
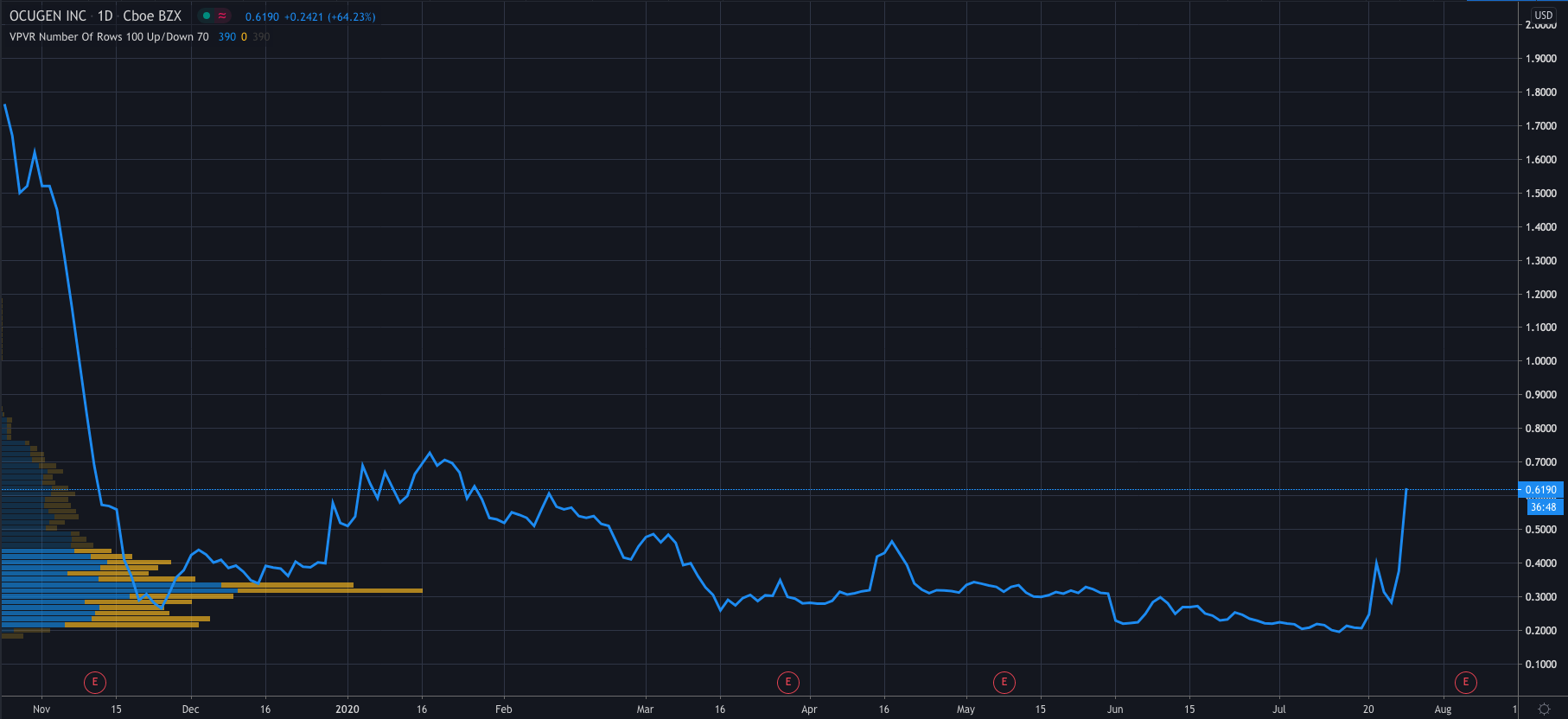
Shares in Ocugen traded as high as 88% earlier in the day, but have dropped off since, now trading at $0.6337 per share, 68% higher than Friday’s close of $0.3769. If its price can close around this level, it will mean Ocugen’s share price has gained 188% in the last month.
The Orphan Drug Act is used to grant special status to a drug or biological product to treat a rare disease or condition upon the request of a sponsor.
According to the company’s press release, the gene therapy product candidate, OCU400 “has the potential to be broadly effective in restoring retinal integrity and function across a range of genetically diverse inherited retinal diseases.”
“Our third ODD for OCU400 from the FDA is an important step towards developing a broad-spectrum treatment for RP and getting a therapy faster to patients who are in desperate need of rescue,” said Dr Shankar Musunuri, Chairman, Chief Executive Officer and Co-Founder of Ocugen.
He continued by saying that “Orphan designation for this indication supports the goal of our Modifier Gene Therapy Platform to treat a variety of inherited retinal diseases with a single gene therapy product. There are currently no approved treatments which slow or stop the progression of multiple forms of RP, which is why we’re excited to have a platform that can potentially address multiple mutations, including mutations in the Rhodopsin gene, with one therapy.”




