Key points:
- Omega Diagnostics is up on news of WHO testing in the CD4 business
- That CD4 business was sold of course
- There's still a possible success payment to come, but that's size limited
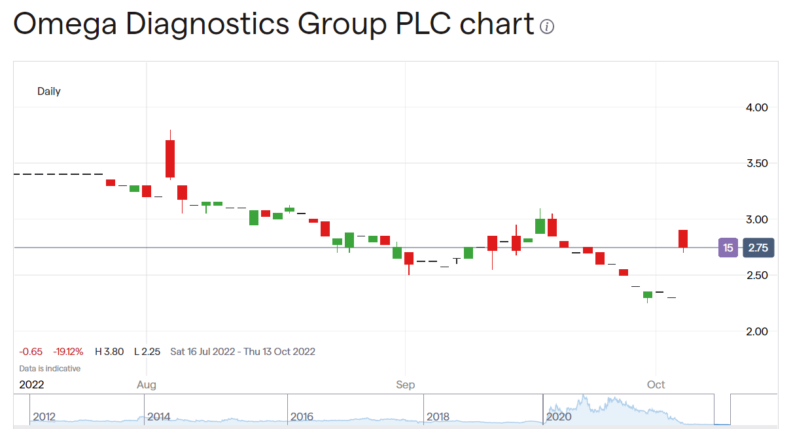
Omega Diagnostics (LON: ODX) is up 17% on positive test results back from the World Health Organisation work. This is good news but needs to be rather tempered by the fact that this is on a product no longer owned by ODX. It has positive cashflow and even profit implications, yes, but we're not in the stonks world of the sky's the limit here.
Omega designs and attempts to gain approval for diagnostics – obviously enough. This does mean that the future of the firm is intimately tied in with the approval process of said medical diagnostics tests. As we saw back with covid there can be many people attempting to design tests and not all of them will get it right. Even CDC itself managed to infect its own test kits, which is regarded as a not sensible thing to do. Omega and Abingdon were tied up in this. Omega did work for Abingdon, Abingdon found it difficult to get payment from the government (on the grounds that the tests didn't quite work) but that was eventually resolved.
There was much excitement in this whole sector in fact, for the fairly obvious reason that we did have a pandemic. As that has faded into the background we're all back to viewing these diagnostics companies on their other work, not on their covid-19 offerings. It's this other work which is the subject of interest today.
Also Read: Five Best Pharmaceutical Stocks to Watch in 2022
In August Omega sold its CD4 business for some £6 million. However, that's not the end of the story. That's a downpayent, rather than the final payment. There's another £4 million that might arrive dependent upon how will that business does in passing subsequent tests on the effectiveness of the, err, tests.
Which is what the subject of today's release is: “These results are in line with management's expectations.” We can leave much of the technical speak out. That's the important point there. This stage of testing hasn't shown up any gremlins, hasn't refuted previous beliefs. A reasonable assumption is therefore that testing will lcontinus along that pathway to full approval.
That £4 million gets closer that is. We do have to grasp how such medical testing systems work though. There are a series of hurdles and failure at any one of them is a disqualification. It's necessary to pass each and every stage of the tests in order to be able to come to market.
So, yes, this is positive for the potential future value of Omega Diagnostics. But it's about that potential value. For we have, as above, simply passed another one of the necessary tests, not actually gone forward to full approval.
We've also that issue of the CD4 business having been sold. The upside here is not that Omega will have a test which can be rolled out globally or anything. The maximum upside is limited to that possible £4 million. So, while this is indeed an advance this is not stonks time.




