The Roivant Sciences (NASDAQ: ROIV) stock price rallied 20.18% after its subsidiary Immunovant reported upbeat Phase I clinical trial data. The data was related to IMVT-1402, a drug candidate targeted at patients with autoimmune diseases.
The trial results indicated that subcutaneous (SC) doses of IMVT-1402 had attained peak Immunoglobulin G (IgG) reductions similar to those previously observed with batoclimab. There was also no decline in serum albumin below baseline nor an increase in low-density lipoprotein cholesterol (LDL-C).
Searching for the Perfect Broker?
Discover our top-recommended brokers for trading stocks, forex, cryptos, and beyond. Dive in and test their capabilities with complimentary demo accounts today!
- eToro Wide range of instruments available to trade – Read our Review
- Vantage High levels of account and deposit protection – Read our Review
- BlackBull 26,000+ Shares, Options, ETFs, Bonds, and other underlying assets – Read our Review
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY
The clinical trial was conducted over four weeks in healthy volunteers to measure the tolerability, safety, pharmacodynamics and pharmacokinetics of IMVT-1402 in healthy adults. The Phase I clinical trial was a double-blind, randomised, placebo-controlled ascending dose study.
The results released today showed that subcutaneously administered doses of IMVT-1402 generated peak Immunoglobulin G (IgG) reductions with close similarity to the results generated by batoclimab. Therefore, IMVT-1402 could be a potential best-in-class neonatal fragment crystallisable receptor (FcRn) inhibitor.
Within the study's single-ascending dose (SAD) part, the IMVT-1402 was administered subcutaneously and demonstrated a consistent reduction in IgG. The drug’s potency was more significant than or similar to batoclimab.
The safety data was also favourable, with all mild or moderate adverse events (AEs). No significant reduction from the baseline in serum albumin or increase in LDL-C was observed at any time intervals (all p>0.05).
The company was also pleased to reveal that the initial multiple-ascending dose (MAD) study results for the 300 mg group were available today. All the data from the MAD study is currently available. The company also recently began the dosing for the 600 mg cohort.
Immunovant also noted that after four weekly doses of 300 mg SC of IMVT-1402, the mean total IgG reduction from baseline in the MAD cohort was 63%. Adverse events emerging from treatment were observed to be mild or moderate in severity.
Pete Salzmann, M.D., Immunovant’s CEO, said: “We are encouraged by the strong pharmacodynamic data observed to date with IMVT-1402. These first-in-human results are consistent with those observed in prior non-human primate studies, and we look forward to sharing additional MAD data in November.”
Roivant Sciences (ROIV) stock price
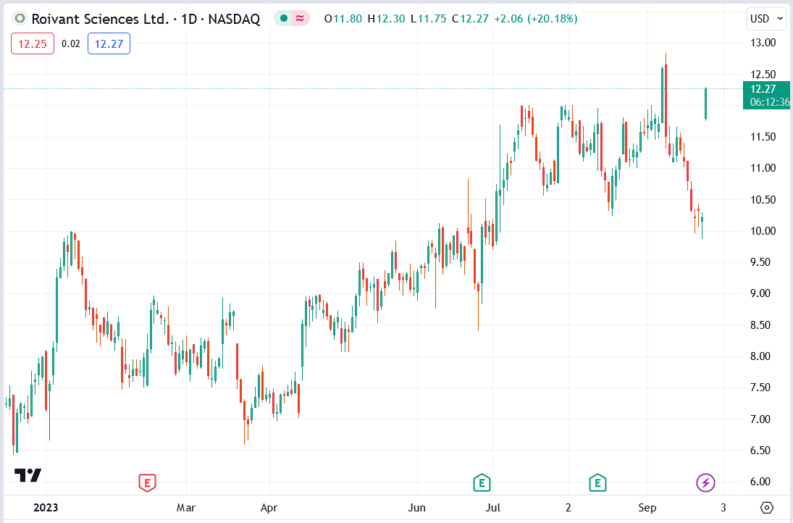
The Roivant Sciences (ROIV) stock price rallied 20.18% to trade at $12.27, rising from Monday’s closing price of $10.21.











