
Shares of Sareum Holdings Plc (LON: SAR) spiked 22.8% higher after the company received the worldwide rights to the FLT3+Aurora kinase inhibitor programme which is meant to help treat immune system overreaction to the coronavirus (COVID-19) and other viral infections.
The biotech company announced late Friday that its licensing partner for FLT3 had decided not to exercise its option to continue the development of the programme, and as a result the programme’s rights will now revert to Sareum.
Sareum had licensed FLT3 to Chinese specialty pharmaceutical company in March 2020 receiving an upfront payment of £50,000; no further payments are due.
The company noted that the licensee had improved the bioavailability of the lead compound in the FLT3 programme, but was unable to achieve the needed levels to meet certain milestone requirements forcing the licensee to terminate the license agreement.
Tim Mitchell, Sareum’s CEO, said: “We will review the data and progress made with the programme by our Chinese partner over the coming weeks and will evaluate options for re-partnering the FLT3 programme. While early studies with our FLT3+Aurora kinase inhibitors were promising, formulation continues to be a challenge”,
Adding:
“Our clear priority remains on advancing our proprietary TYK2/JAK1 programmes and, in the near-term, on completing the preclinical work needed for the first Clinical Trial Application during the first quarter of the year. Achieving this important milestone will be a key step in generating shareholder value from our TYK2/JAK1 pipeline.”
Sareum share price
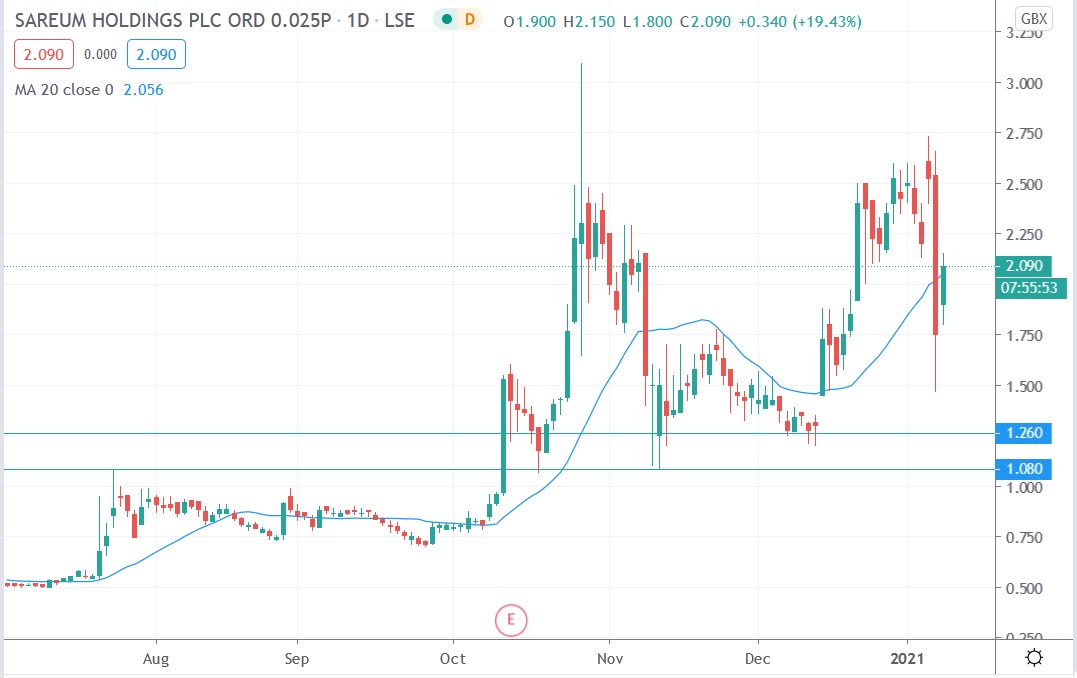
Sareum shares exploded 22.85% higher to trade at 2.15p having rallied from Friday’s closing price of 1.75p.




