Shares of Synairgen plc (LON: SNG) surged 13.6% after its respiratory COVID-19 drug SNG001 was included in the US ACTIV-2 Phase II trial targeting patients with the coronavirus whose symptoms do not warrant hospitalisation.
The US Government funded the ACTIV Partnership under the “Operation Warp Speed” program designed to speed up the development of the most promising treatments and vaccine candidates for COVID-19.
The phase II trial will recruit up to 220 participants from the United States and be administered in a home-based setting and test SNG001 against a placebo. Positive results from the phase II study will lead to a phase III trial.
SNG001 is an inhaled interferon beta-1a treatment proven effective in treating hospitalised patients with severe COVID-19 symptoms.
Synairgen recently launched the phase III trial of SNG001 on 12 January 2021 to prove its effectiveness in treating coronavirus patients in a hospital setting.
Richard Marsden, CEO of Synairgen, said: “The inclusion of our inhaled interferon beta-1a treatment in the US Government-funded ACTIV-2 trial reflects the strong interest that our Phase II data has generated and the Company’s strong belief that this drug could play a vital role in the treatment of COVID-19. As an inhaled treatment, SNG001 offers ease of use that makes it possible for patients to administer it conveniently at home, reducing the risk of virus transmission during hospital visits and relieving the major logistical strain on healthcare systems.”
Adding:
“At-home treatments also have the potential to be taken much earlier in the course of the illness, preventing the progression of the virus in the lower respiratory tract and, in the scenario where hospitals are at capacity, treating patients in the home setting could be the only option. We hope to gain valuable data from this trial, which will aid us in progressing approval of SNG001 as a treatment for COVID-19.”
Synairgen share price
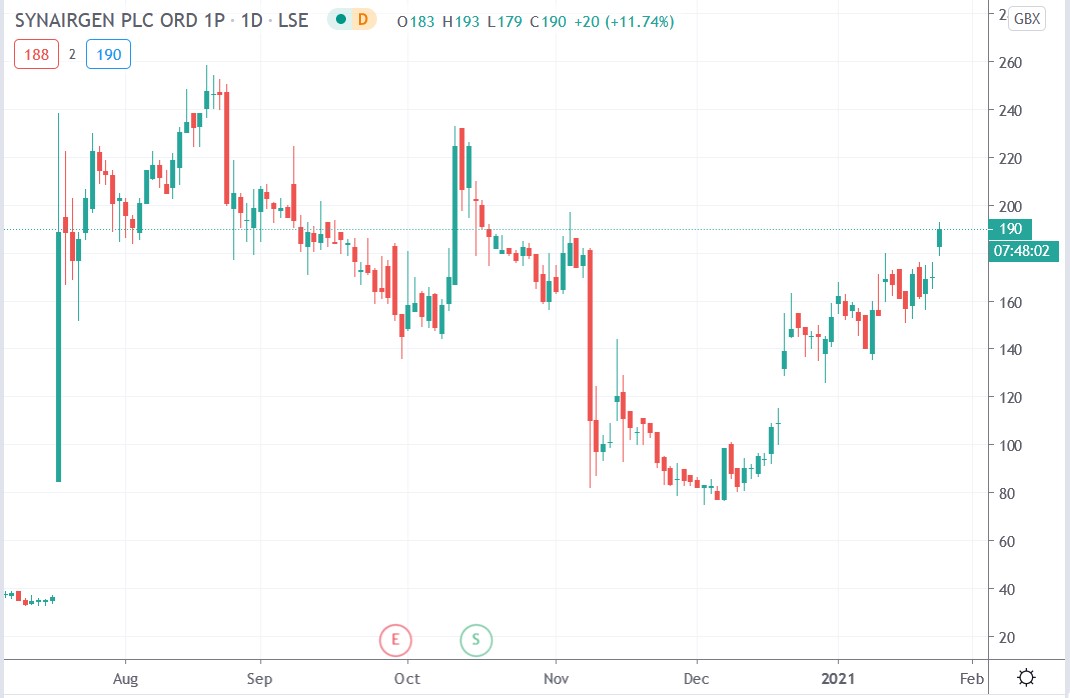
Synairgne share surged 13.52% to trade at 193p having rallied from Friday’s closing price of 170p.




