vTv Therapeutics Inc (NASDAQ: VTVT) stock rose 31.6% after releasing the results of a multiple ascending dose study for HPP737, an oral PDE4 inhibitor drug that treats psoriasis.
The biotech company revealed that HPP737 demonstrated a favourable safety and tolerability profile without dose-limiting gastrointestinal side effects.
Today’s results build on data from the previous multiple ascending dose study and support the advancement of HPP737 into Phase 2 trials in patients with moderate to severe psoriasis.
vTv has already held a successful pre-IND meeting with the U.S. Food and Drug Administration’s Dermatology and Dentistry division, supporting the planned IND submission for a Phase 2 study in moderate to severe psoriasis patients.
The trial had 12 participants in two groups randomly given 15mg and 20mg doses of HPP737 or placebo orally once daily over 14 days.
None of the participants had adverse events due to the treatment, which is why vTv believes that it can move forward to develop the drug to achieve anti-inflammatory and anti-psoriatic responses without the significant safety issues of other PDE4 inhibitors.
Investors celebrated the announcements, as evidenced by the premarket rally. Many expect the FDA to okay a phase 2 study of HPP737, which could be a massive milestone for the company.
As it stands, there is little evidence to suggest that the FDA may reject a phase 2 trial; hence, the market’s exuberance is justified in this case.
*This is not investment advice.
vTv Therapeutics stock price.
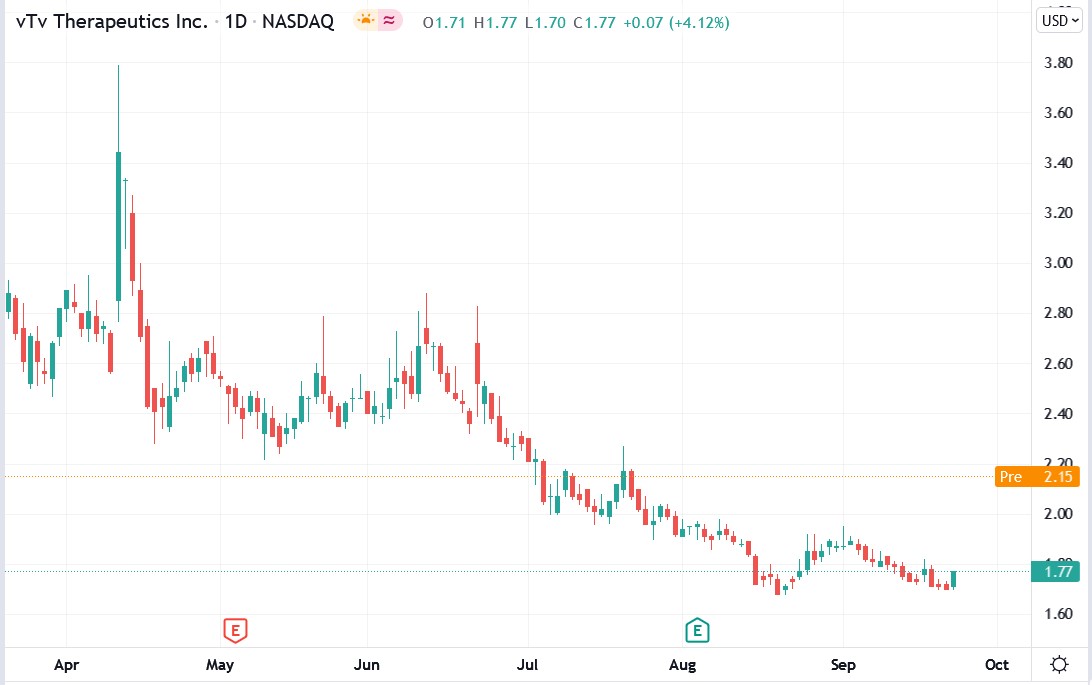
vTv Therapeutics stock rallied 31.63% premarket to trade at $2.33, rising from Thursday’s closing price of $1.77.




